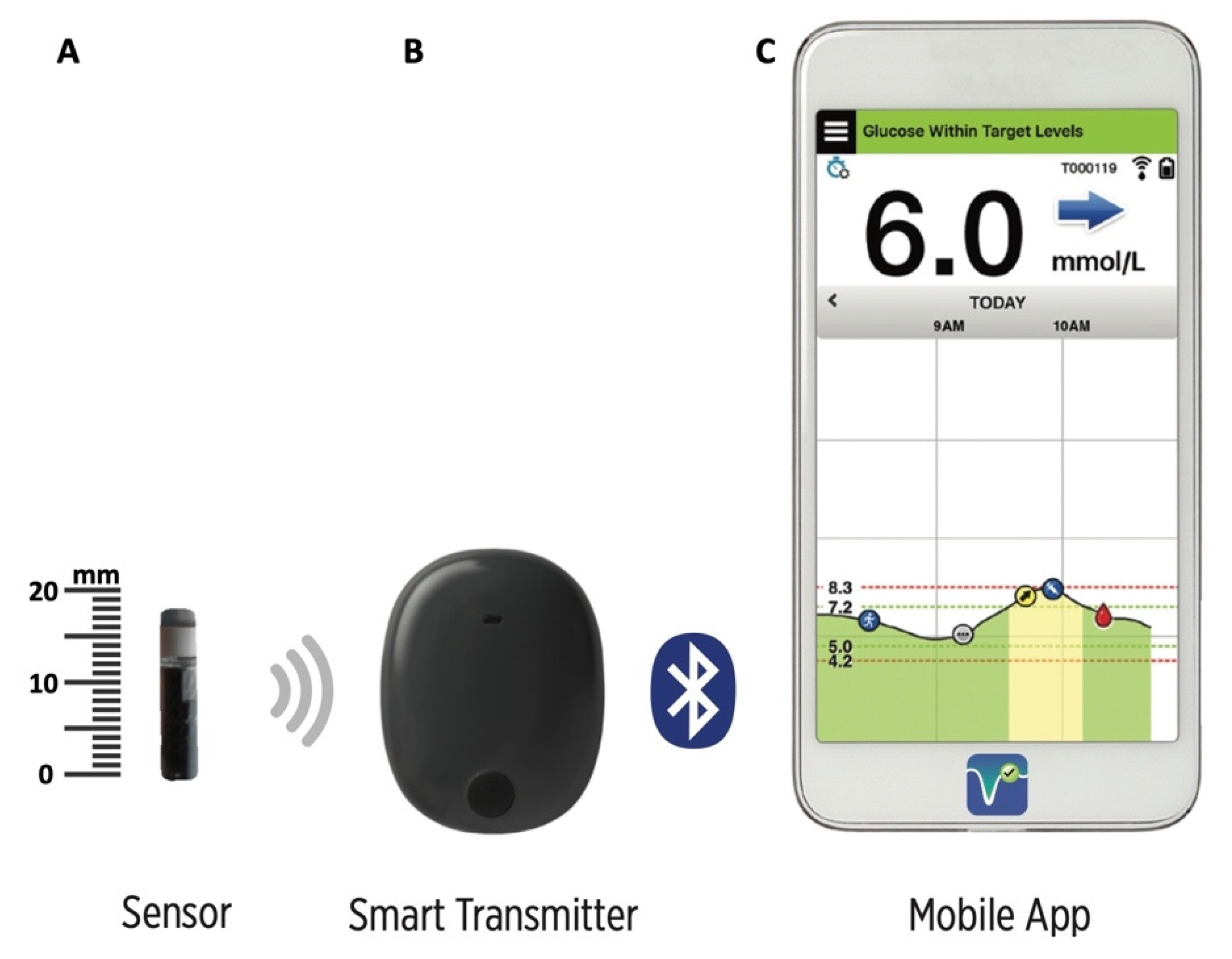
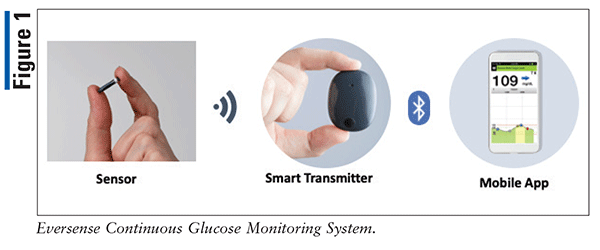
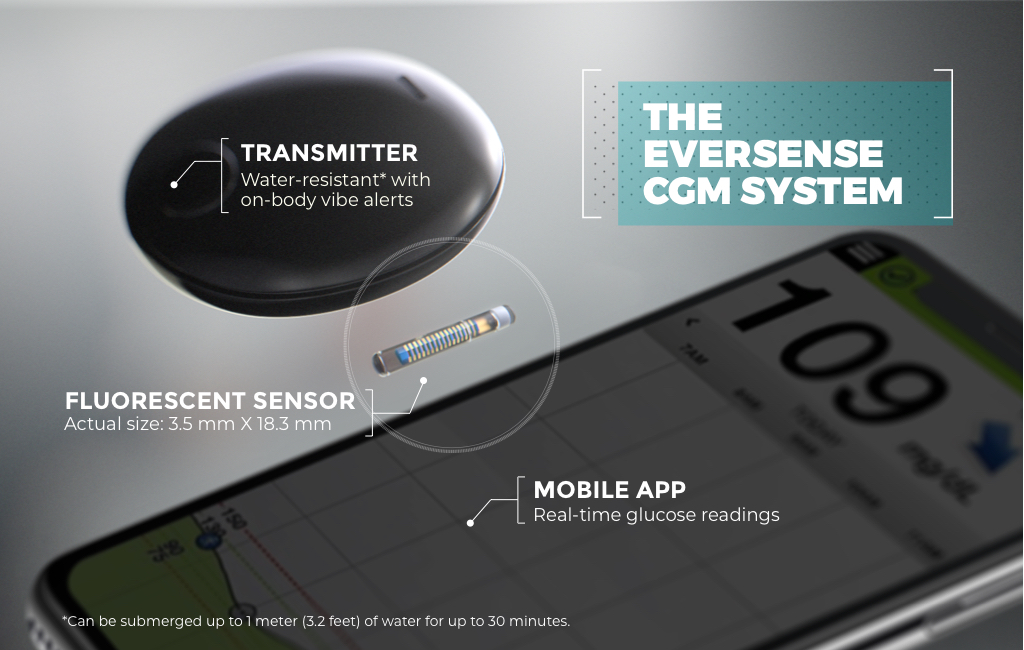
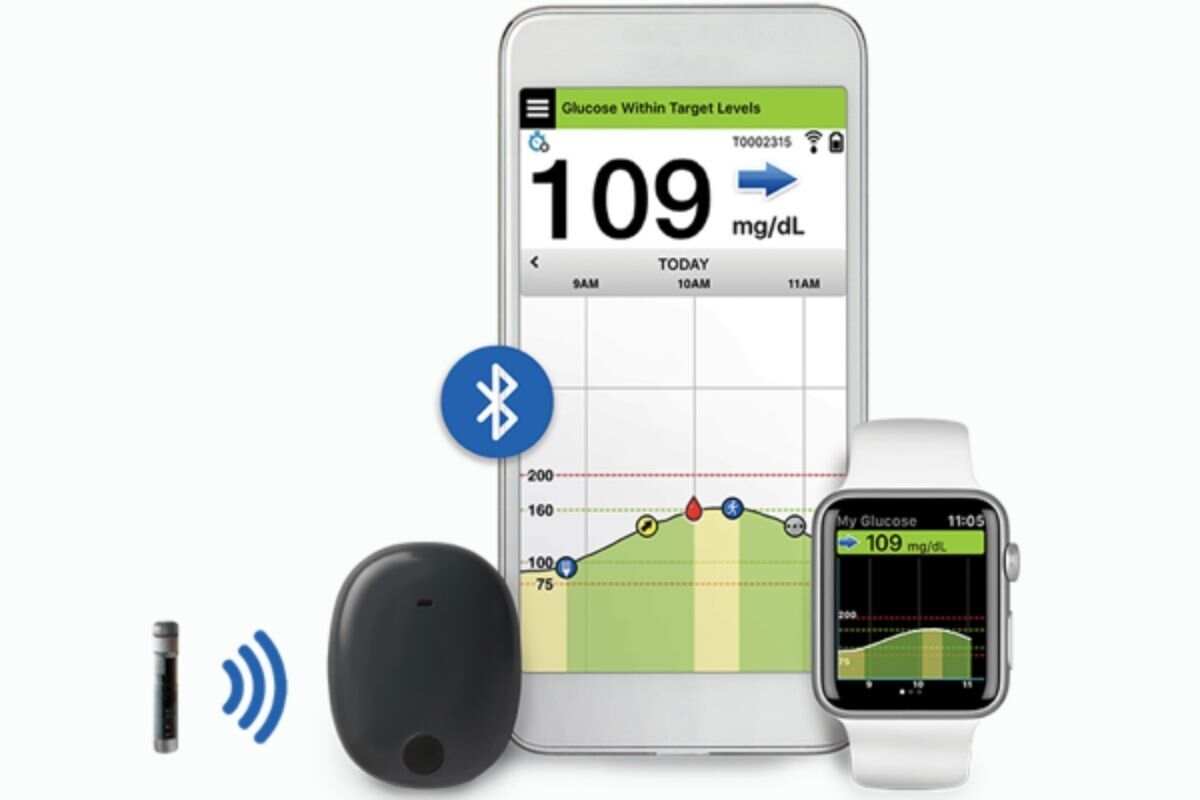
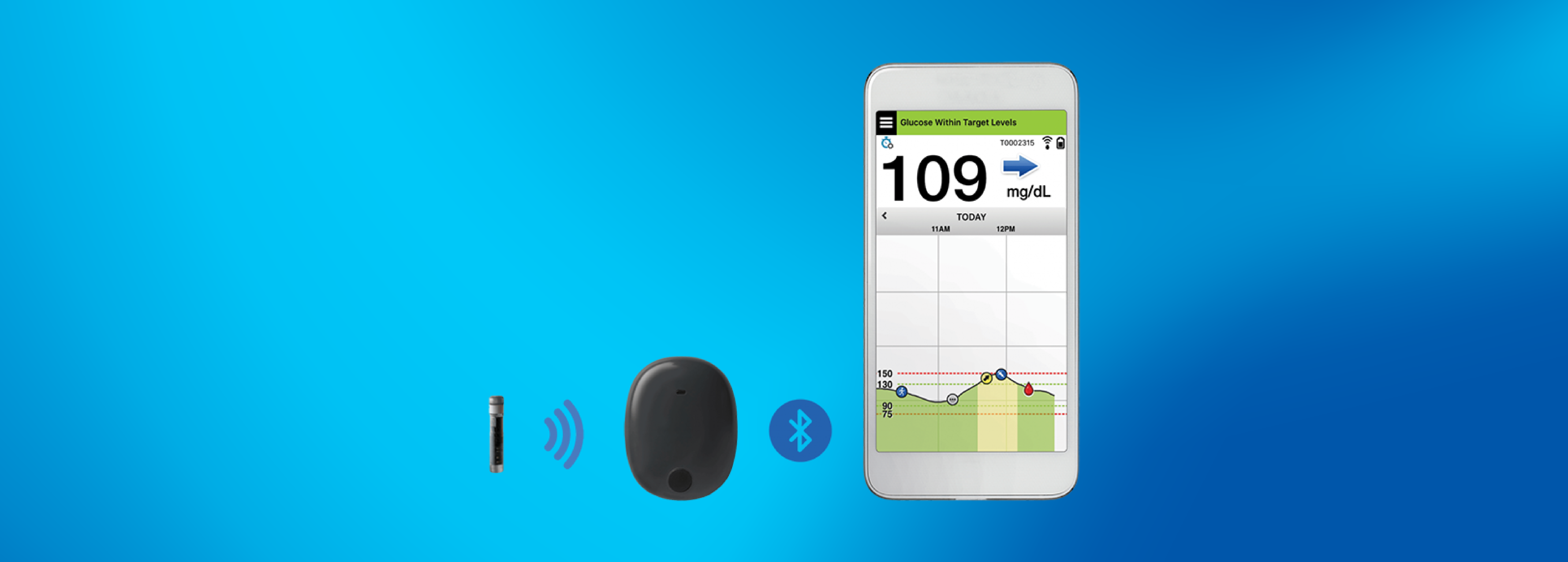
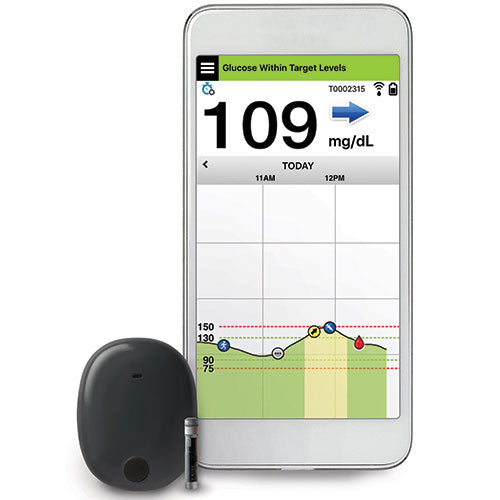
FDA approves Eversense E3 6-month continuous glucose monitor that requires fewer fingerstick blood glucose measurements - NotebookCheck.net News

Continuous Glucose Monitoring (CGM) System for Diabetes Management Market Growth Analysis Report 2023-2030 |Medtronic,

Senseonics' CE Mark for Eversense continuous glucose monitoring system - Today's Medical Developments

Senseonics' recently approved implantable glucose monitor wins real-world accuracy study | Fierce Biotech